One of the most known examples of natural selection in action is the evolution of the peppered moth (Biston betularia), the rapid replacement of the light-colored form of the moth (typica) by a dark-colored form (carbonaria) (Fig. 1) during 1800s in Britain. The first live specimen of the carbonaria form was found in 1848 and its frequency had increased drastically until late 1800s. In 1895, 98% of the moth population in Manchester was the carbonaria form (reviewed in Clarke et al., 1985). Such a phenomenon 36 years after the publication of Darwin’s On the Origin of Species, attracted biologists’ attention. J.W. Tutt first proposed “Differential bird predation hypothesis” in 1896, which is confirmed by a series of experiments by Kettlewell during mid 1950s (reviewed in Cook and Saccheri, 2013). The hypothesis states that the industrial revolution in Britain resulted in blackened trees by soot, so that birds can easily spot light-colored moths on soot-darkened trees while dark-colored moths are camouflaged. However, genetic events giving rise to carbonaria phenotype remained elusive until recently. Researchers from University of Liverpool and Wellcome Trust Sanger Institute now reported in Nature that the mutation causing the peppered moth industrial melanism is the insertion of a large, tandemly repeated transposable element into first intron of gene cortex.
Figure 1. The dark-colored form, carbonaria (top) and the light-colored form, typica (bottom) of Biston betularia
The term industrial melanism refers to darkening of species in response to pollutants. It is widespread in many Lepidoptera species (moths and butterflies). Initial experiments identified that melanism in Biston betularia is determined by a single locus dominant allele (reviewed in Cook and Saccheri, 2013). However, the molecular identity of the gene determining the melanism in peppered moths was completely unknown. In order to determine the gene identity, van’t Hof and Saccheri looked for associations between genetic polymorphisms within sixteen genes previously implicated in melanisation pattern differences in other insects and the carbonaria morph by the candidate gene approach (van’t Hof and Saccheri, 2010). However, this earlier study showed that the carbonaria gene is not a structural variant of a canonical melanisation pathway gene. One year after the failure of the candidate gene approach, Saccheri group constructed a linkage map to identify the chromosomal region containing the carbonaria–typica polymorphisms. In 2011, they coarsely localized the carbonaria locus to a <400-kilobase region orthologous to a segment of silkworm (Bombyx mori) chromosome 17 (van’t Hof et al., 2011). However, what the gene is and what it does was still a mystery.
The same group now reported that they have found the gene and the mutation event causing the industrial melanism in Biston betularia (van’t Hof et al., 2016). By using a larger population sample and more closely spaced genetic markers, they narrowed down the carbonaria candidate region to ~100 kb region in Biston betularia genome. The candidate region is the orthologue of Drosophila cortex (cort) gene. As a distant member of the Cdc20 protein family, Drosophila cort gene encodes for a cell-cycle regulator and is shown to be important in regulating oocyte meiosis (Chu et al., 2001), but it is not involved in wing patterning or development. Unlike Drosophila cort, two of multiple alternative first exons (1A and 1B) in Biston betularia cortex are strongly expressed in developing wing disks. In addition, cortex gene has a very large first intron and eight non-first exons.
After identification of the gene, authors compared one carbonaria to three typica haplotypes to identify the first set of carbonaria specific polymorphisms. This initial alignment revealed 87 melanisation candidate polymorphisms concentrated within the large first intron of the gene. However, natural selection increases not only the frequency of the favored allele in carbonaria but also the frequency of the neutral alleles linked to the causal allele. In an earlier study, they have also shown that Biston betularia melanism was originated from a single recent mutation (van’t Hof et al., 2011). Having screened more typica individuals, they further eliminated rare variants and were eventually able to find one polymorphism unique to carbonaria, a very large insert in the first intron of the gene.
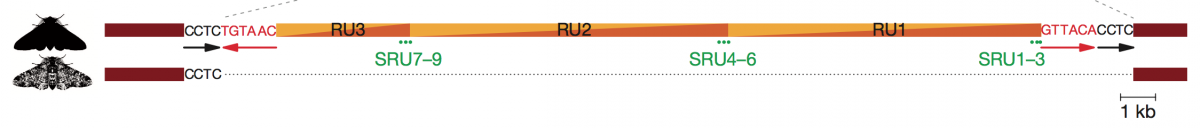
The size of the causative large insert is 21,925 nucleotides long and is composed of a roughly 9-kb essentially non-repetitive sequence. The nature of the insert indicated that it is a class II transposable element (TE) – DNA transposon. The transposition of class II TEs are catalyzed by transposases that cut the DNA at the target site in a staggered fashion producing 5′ or 3′ DNA overhangs that are duplicated after transposition. Another hallmark of class II TEs is short inverted repeats at two ends of TE. Sequence analysis of the insert and comparison with the typica haplotypes revealed that both short inverted repeats (6 bp) and duplication of the target site (4 bp) are present in the carbonaria insert (Fig. 2).
Figure 2. The structure of the insert, shown in the carbonaria sequence, corresponds to a class II DNA transposon, with direct repeats resulting from target site duplication (black nucleotides) next to inverted repeats (red nucleotides). Typica haplotypes (lower sequence) lack the 4-base target site duplication, the inverted repeats and the core insert sequence. The transposon consists of ?9 kb tandemly repeated two and one-third times (repeat unit (RU)1–RU3), with three short tandem subrepeat units (green dots, SRU1–SRU9) within each repeat unit.
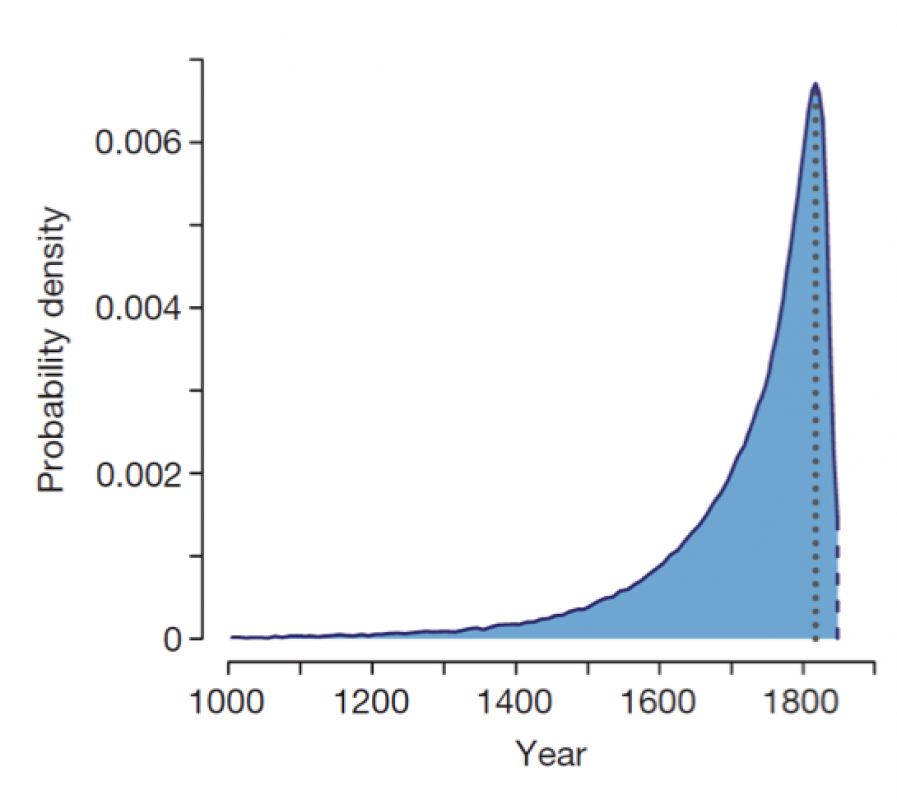
To estimate the age of mutation event, the authors looked at 200 kb either side of the carb-TE insert. The idea is to track recombination events that have eroded the ancestral carbonaria haplotype. Given the ancestral state of carbonaria haplotype and recombination rate, how many years do we need to explain the observed haplotypes that are shuffled version of the ancestral one? Simulations based on this assumption predicted the most likely date of the mutation as 1819, shortly before it was first seen in the wild (1848) (Fig. 3).
Figure 3. Probability density for the age of the carb-TE mutation inferred from the recombination pattern in the carbonaria haplotypes (maximum density at 1819 shown by dotted line; first record of carbonaria in 1848 shown by dashed line).
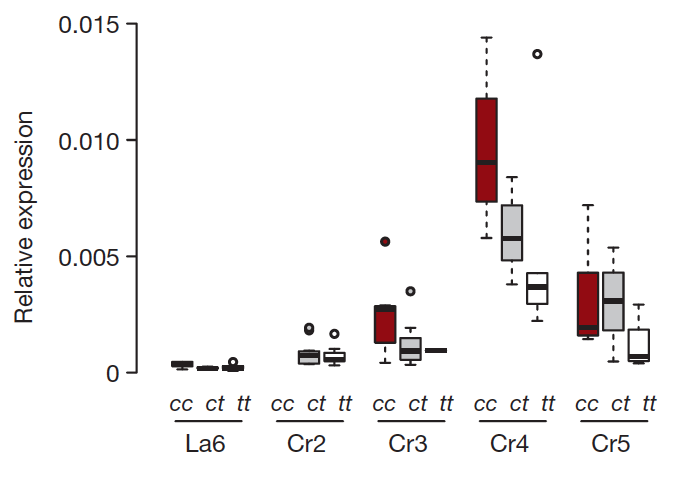
The next question is how the carbonaria – TE leads to the melanisation of Biston betularia. TEs localized in introns effects the expression of the gene through several mechanisms. In order to test this possibility, first they checked tissue-specific expression of cortex splice isoforms and alternative first exons. They have identified two first exons, 1A and 1B, which are expressed highly in developing wing discs. Comparison of the abundance of 1A and 1B-initiated full transcripts between different genetic backgrounds (homozygous carbonaria – c/c, homozygous typica – t/t, and heterozygous individuals – c/t) revealed that 1B expression is significantly higher in carbonaria background (c/c > c/t > t/t) (Fig. 4), whereas 1A-initiated full transcript does not show a significant difference between genotypes. In addition, cumulative expression of all splice-isoforms increases starting from the sixth larval instar (La6) until day 6 prepupa (Cr6) with highest value on day 4 prepupa (Cr4). Surprisingly, a phase of rapid wing disc morphogenesis also occurs in the same time interval, possibly indicating a function of cortex in wing pattern melanisation.
Figure 4. Tukey plot for relative expression of cortex 1B full transcript in developing wings of the three carbonaria-locus genotypes (c/c, c/t and t/t) produced within the progeny of a c/t x c/t cross. Genotypes differ significantly for the transcript (P < 0.001)
As mentioned earlier, Drosophila cort encodes for a distant member of the Cdc20 protein family (Chu et al., 2001). Members of the Cdc20 protein family activate an “E3” ubiquitin ligase, the anaphase-promoting complex (APC) and present its substrates. APC then ubiquitinates presented cell-cycle proteins, causing their degradation. This proteolysis destroys a panel of proteins including cyclins, allowing the cell cycle to progress. Degrons, short linear motifs located anywhere in the protein, are important for substrate recognition in proteolysis. A single shared site in lepidopterans and non-lepidopterans cortex binding the same degron sequence is also predicted for both 1A and 1B full isoforms, indicating a shared function of cortex between D. melanogaster and B. betularia. However, we still need further evidence to understand the exact connection between cell-cycle protein degradation and melanisation.
In conclusion, we now know that the industrial melanism mutation event in British peppered moth is the insertion of a large, tandemly repeated, transposable element into the first intron of the gene cortex. Although we still do not know the molecular mechanisms connecting cortex gene and the melanism in peppered moths, the discovery of causative mutation as a transposable element is breakthrough in the peppered moth story. In addition, it provides a spectacular evidence for the importance of transposable elements in adaptive evolution.
References:
Chu, T., Henrion, G., Haegeli, V., and Strickland, S. (2001). Cortex, a drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/fizzy protein family. Genesis 29, 141–152.
Clarke, C.A., Mani, G.S., and Wynne, G. (1985). Evolution in reverse: clean air and the peppered moth. Biol. J. Linn. Soc. 26, 189–199.
Cook, L.M., and Saccheri, I.J. (2013). The peppered moth and industrial melanism: evolution of a natural selection case study. Heredity (Edinb). 110, 207–212.
van’t Hof, A.E., Edmonds, N., Dalíková, M., Marec, F., and Saccheri, I.J. (2011). Industrial melanism in British peppered moths has a singular and recent mutational origin. Science 332, 958–960.
van’t Hof, A.E., Campagne, P., Rigden, D.J., Yung, C.J., Lingley, J., Quail, M.A., Hall, N., Darby, A.C., and Saccheri, I.J. (2016). The industrial melanism mutation in British peppered moths is a transposable element. Nature 534, 102–105.
van’t Hof, A.E., and Saccheri, I.J. (2010). Industrial melanism in the peppered moth is not associated with genetic variation in canonical melanisation gene candidates. PLoS One 5.