Introduction
Environmental pollution is a widespread problem that living organisms have to contend with on a global scale. In contaminated sites especially, wild populations undergo intense selective pressure that may result in phenotypic adaptations to pollutants (Hendry et al., 2008). The scientific article (Reid et al., 2016) discussed in this blogpost explores the genetic mechanisms that have allowed the rapid adaptation to industrial pollutants in wild Atlantic killifish populations.
Results
The genomic landscape of the killifish populations
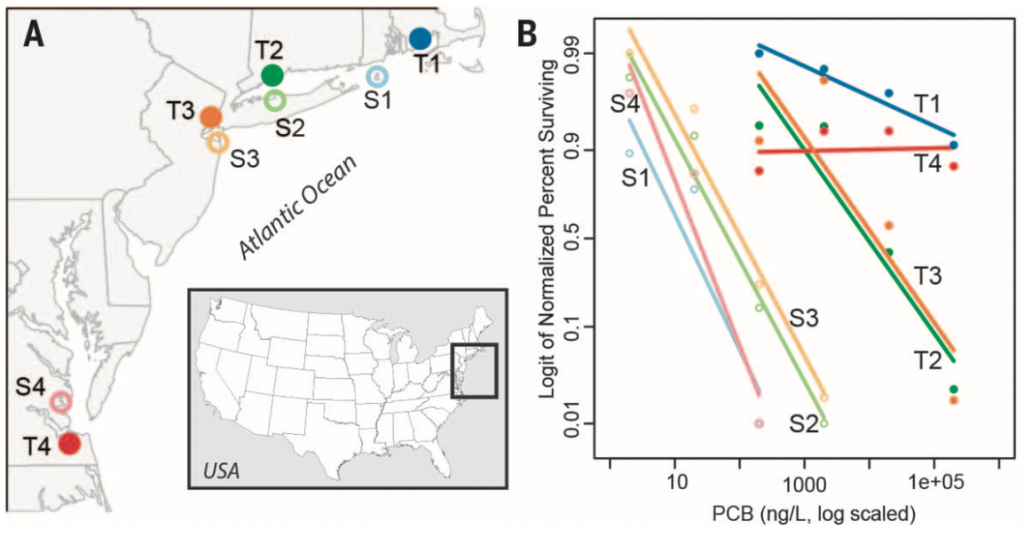
Atlantic killifish (Fundulus heteroclitus) are non-migratory fish that are abundant along the US east coastline (Fig. 1A). Some killifish populations show inherited resistance to lethal levels of industrial pollutants in sites that have been contaminated for decades. For instance, the authors show that the percentage of larva that survive in increasing concentrations of a highly toxic pollutant called PCB 126, is higher in tolerant populations compared to the sensitive populations (Fig. 1B). To understand the genetic adaptations underlying the rapid adaptation to polluted sites in killifish populations, the authors sequenced the complete genomes from eight populations. Four tolerant populations that reside in highly polluted sites were sampled. Each one was paired with a sensitive population from a nearby site (Fig. 1A). The authors combined these genomic data with corresponding RNA sequencing (RNA-seq) to identify unique and shared pathways among tolerant populations as well as to uncover adaptive evidence in the populations.
The genomes from 43-50 individuals from each population were sequenced. One pair of tolerant and sensitive populations (T1 and S1) were sequenced to 7-fold coverage, while the remaining populations, to 0.6-fold coverage. These data indicate that the populations’ genetic variation is strongly by their geographical locations. Meanwhile, all tolerant and sensitive pairs of populations share the most similar genomic backgrounds and have low Fst values between them (0.01 – 0.08). Additionally, tolerant populations show a lower genome-wide nucleotide diversity (?) along with a positive-shifted Tajima’s D. Thus, the authors conclude that tolerant populations have recently and independently diverged from local ancestral populations.
Signatures of convergent evolution in tolerant killifish populations
To identify genomic regions responsible for conferring pollution tolerance in killifish, the authors scanned the populations’ genomes looking for signals of selective sweeps in 5 kb sliding windows. Candidate regions were defined as those showing low values of genetic diversity and a skewed allele frequency spectrum (0.1% tails of ? and Tajima’s D, respectively) as well as high allele frequency differentiation (99.9% tails for Fst). Each tolerant population showed prevalent selection signatures compared to their sensitive counterparts (as seen by ? and Fst). Most of these outlier regions are small (52 – 69 kb, up to ~1.8 Mb) and specific to each tolerant population. Nevertheless, the highest ranked outlier regions are shared between tolerant populations (Fig. 2A). The shared outlier regions harbour genes involved in the aryl hydrocarbon receptor (AHR) signaling pathway (AHR2a, AHR1a, AIP, and CYP1A) (Fig. 2B). These results suggest repeated convergent evolution of pollutant tolerance in the sampled killifish populations.
The authors then tested whether the genes located in outlier regions showed distinct expression profiles in tolerant killifish. Individuals from sensitive and tolerant populations were reared in a common, clean environment for two generations. Following this, embryos were challenged with the toxic pollutant PCB 126 and RNA was collected ~10 days post fertilization. Indeed, AHR-regulated genes were less induced in individuals from tolerant populations (Fig. 2C). Concomitantly, AHR-regulated genes were enriched (P < 0.0001) in the set of genes that were up-regulated in response to PCB 126 treatment in sensitive populations exclusively. Notably, some of the dominant pollutants at the sampled “T” sites bind AHR. Also, aberrant AHR signalling leads to embryo and larval lethality (Pohjanvirta, 2011). The authors thus conclude that the AHR signalling pathway is a key and repeated target of natural selection in polluted sites given the multiple, independent “desensitizing” events in tolerant killifish populations.
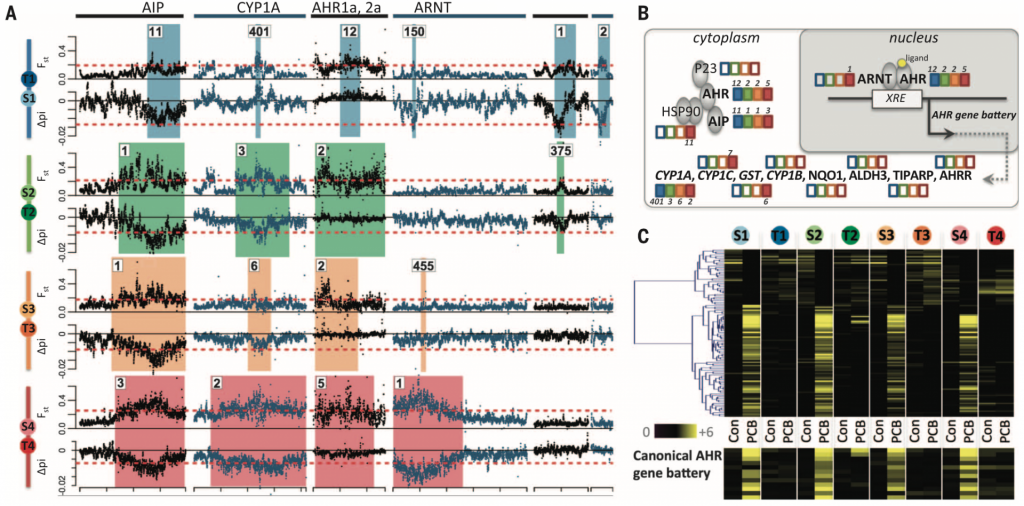
It is important to note that genome sequencing coverage seems to have an effect on the ranking of outlier regions. For instance, the regions that contain key AHR-signalling genes (AIP, CYP1A, AHR1a/2a, and ARNT) are very highly ranked in low-coverage populations whereas they are lowly ranked in the high-coverage population pair (T1-S1). Given that outlier regions are ranked based on Fst and nucleotide diversity, these measures must be impacted by low genome sequencing coverage. It would be interesting to determine the ranking of the outlier regions if the other populations were sequenced to higher coverage. However, despite being lowly ranked, these regions are classified as outliers in all four population pairs, giving strength to the argument that impaired AHR-signalling is key to pollution tolerance.
In-depth analysis of genetic variants in tolerant populations
There is evidence for selection of AHR pathway genes in tolerant killifish populations. Tolerant populations harbour distinct deletions spanning AHR2a and AHR1a. On the contrary, individuals from their sensitive counterparts are almost completely devoid of such mutations. Furthermore, RNA-seq data revealed the expression of a chimeric transcript, part AHR2a, part AHR1a in T4 individuals. Meanwhile, AIP (a regulator of AHR stability and cellular localization) is found within a region showing the strongest signals of selection that is shared between all tolerant populations. CYP1A (a transcriptional target of AHR) is also in located in top-ranking outlier regions in all tolerant populations (except for T1 where the region is ranked #401). Interestingly, CYP1A duplications are found in high frequencies in tolerant populations, without a concomitant increase in expression. The authors hypothesize that CYP1A duplications may function as a dosage-compensation mechanism in tolerant populations with impaired AHR signalling because it has been reported that AHR knockout decreases CYP1A expression in rodents (Schmidt et al., 1996). Finally, other AHR-related genes lie within population-specific outlier regions such as the tandem paralogs AHR1b and AHR2b in T3 and T4 and five other AHR pathway genes in T4. Together these observations led the authors to conclude that AHR pathway genes are indeed common and repeated targets of selection, a clear example of convergent evolution.
Genes outside of the AHR signalling pathway are also targets of selection. For example, two genes that are implicated in AHR-independent cardiotoxicity (KCNB2 and KCNC3) are within outlier regions in T4, where such cardiotoxic pollutants are abundant. Additionally, the authors found adaptations that may compensate for the potential costs of pollutant tolerance. AHR signalling is interconnected with multiple other pathways, such as estrogen and hypoxia signalling, as well as cell cycle and immune system regulation (Beischlag et al., 2008). Consequently, estrogen receptor 2b lies within an outlier region in T2, while estrogen receptor-regulated genes are enriched in the gene set of the outlier regions in all tolerant populations (P < 0.001). Furthermore, the estrogen receptor is inferred as an upstream regulator of differentially expressed genes between tolerant and sensitive killifish (Fig. 2C). Alternatively, the hypoxia-inducible factor 2? is in an outlier window in T3, and interleukin and cytokine receptors are in outlier regions in T4. Thus, the authors highlighted the possibility that compensatory adaptation selection may be common following rapid adaptive evolution.
Conclusions
In this article, we can appreciate that genetic adaptations to pollution in wild killifish populations are complex. The authors attribute this to two main factors. Firstly, sites are contaminated with a complex mix of pollutants. This may affect how the AHR- and other pathways are impacted. Therefore, adaptations in multiple pathways, at different genetic levels, may be necessary for tolerance to diverse pollutant mixes to arise. Second, AHR pathway genes are interconnected with other gene-regulatory pathways, thus these genes’ functions may be impaired upon aberrant AHR signalling, It follows that adaptations that compensate these genes’ functions may also be selected for in tolerant populations.
The authors argue that their data clearly reveal signals of convergent evolution. The AHR pathway genes are shown to be repeated targets of selection in distinct pollutant-tolerant killifish populations. This also suggests molecular constraints in the adaptation to pollution. However, in spite of this, multiple variants were favoured in different tolerant populations. The authors say that their data show evidence of selection of preexisting common variants in multiple tolerant populations. In other words, it seems that soft sweeps have been important for the emergence of pollutant tolerance in killifish. This conclusion is supported by several lines of evidence: 1) the sensitive-tolerant populations are genetically close, which suggests that the selected variants were part of the standing variation, 2) sensitive populations have some of the variants that tolerant populations have, and finally 3) these fish are low dispersal. Interestingly, the authors point out that Atlantic killifish have large population size and a wide range of standing genetic variation. These little critters were not only the first space-going-fish, they are one of the most genetically diverse vertebrates, which positioned them well to evolve pollutant-tolerance.
However, it is important to realize that not all species are as well poised to adapt to ever-changing, increasing pollution in their habitats. Research like this gives us key information on how natural populations are dealing with our pollution. The best chance we can give all life forms on earth is to curb or rates of worldwide pollution. Luckily, it is on our power to do so!
References
- Beischlag, T. V., Morales, J. L., Hollingshead, B. D., & Perdew, G. H. (2008). The aryl hydrocarbon receptor complex and the control of gene expression. Critical Reviews in Eukaryotic Gene Expression, 18(3), 207–250. http://doi.org/10.1615/CritRevEukarGeneExpr.v18.i3.20
- Hendry, A. P., Farrugia, T. J., & Kinnison, M. T. (2008). Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology, 17(1), 20–29. http://doi.org/10.1111/j.1365-294X.2007.03428.x
- Pohjanvirta, R. (2011). The AH Receptor in Biology and Toxicology. Wiley, Hoboken, NJ.
- Reid, N. M., Proestou, D. A., Clark, B. W., Warren, W. C., Colbourne, J. K., Shaw, J. R., et al. (2016). The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science (New York, N.Y.), 354(6317), 1305–1308. http://doi.org/10.1126/science.aah4993
- Schmidt, J. V., Su, G., Reddy, J. K., Simon, M. C., & Bradfield, C. A. (1996). Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proceedings of the National Academy of Sciences of the United States of America, 93(13), 6731–6736.
